
The Breakthrough of RYR, with Proven Clinical Trials and over 130 Publications for Metabolic Health
Market leadership in Taiwan red yeast rice and probiotic markets
R&D Personnel Team
Leadership team with proven track record of driving success globally
Leadership team with proven track record of driving success globally
ANKASCIN 568 is extracted from fermented products of patented functional red yeast strain (Monascus purpureus NTU 568). Unlike conventional manual fermentation with derivative quality instability, this premium ingredient is the only red yeast fermented extract manufactured using an exclusive automated solid state fermentation technique. ANKASCIN 568 is FREE of risky lovastatin/monacolin K but contains high levels of another two new active compounds (Monascin and Ankaflavin). Safety and functions have been verified through plenty of animal and clinical studies. In 2015, this ingredient was honorably reviewed and accepted by the US FDA as a NEW DIETARY INGREDIENT (NDI), which made it the only eligible red yeast ingredient on the US market that could legally apply claims and clearly specify the contents of active compounds. Therefore, it is greatly suitable for formulation of dietary supplements and fortified foods.


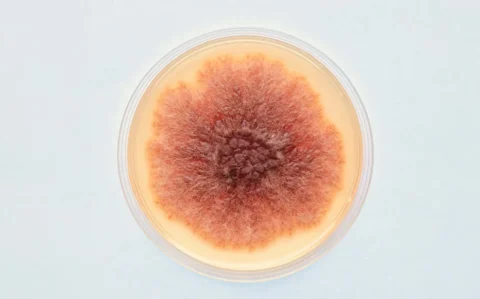
Monascus purpureus NTU 568 is a patented functional red yeast strain isolated and studied for more than ten years by the research team of Professor Tzu-Ming Pan from National Taiwan University. Its fermented products contain high levels of two active compounds: Monascin and Ankaflavin. Science basis is perfectly supported by more than 120 related SCI publications, including a large number of animal and clinical studies verifying its effects on regulation of blood lipids, blood glucose, blood pressure, prevention and treatment of Alzheimer’s Disease, etc. It was also certified with plenty of patents from the USA, EU, Canada, Japan, Australia, China, Korea, Singapore, Taiwan, etc.
Active Compound Contents
Monascin ≥ 28 mg/g
Ankaflavin ≥ 9 mg/g
Daily Dosage
110 mg or 220 mg (per US FDA NDI acknowledgment)
Appearance
Red powder
Package
2 kg / aluminum foil bag
Storage & Shelf Life
2 years sealed at 25°C
Safety Tests
Special Status
US FDA New Dietary Ingredient (NDI) approved (Report #1071: fda_report001)
Patents & Research
Patents granted in USA, EU, Canada, Japan, Australia, China, Taiwan, Singapore, Korea
Scientific Support
Backed by 120+ SCI publications including animal and clinical studies





























