
The Breakthrough of RYR, with Proven Clinical Trials and over 130 Publications for Metabolic Health
Probiotic and its exosome for cognitive health
R&D Personnel Team
Leadership team with proven track record of driving success globally
Leadership team with proven track record of driving success globally
ExoBDNF™ contains the functional strain Pediococcus acidilactici SWP-CGPA01, which is a legally edible probiotic species in EU, Canada, China, and Taiwan. Safety and functions have been verified through plenty of scientific studies. It possesses strong tolerance to gastric acid and bile salts so there is no need for microencapsulation. As a food ingredient and/or dietary ingredient, its concentration is up to more than 10 billion CFU/g and very low water activity (< 0.2) contributes to its high stability. Thus, it is very suitable for formulation of dietary supplements and fortified foods.

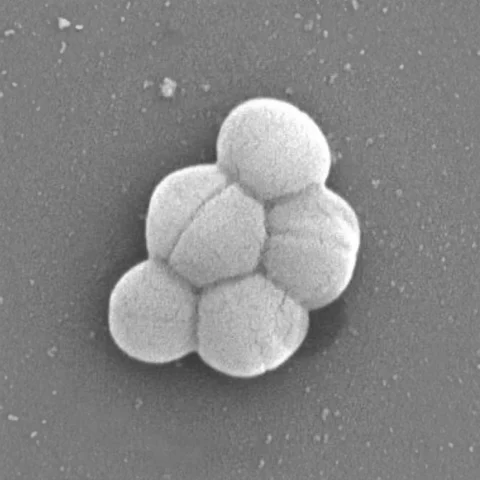
Pediococcus acidilactici SWP-CGPA01 is a functional strain of lactic acid bacteria aimed at promoting both gut and brain health. Its entire genome has been fully sequenced, and a third-party certification agency has confirmed its non-pathogenic properties. The strain is recommended for addressing health concerns associated with aging and microbiota dysbiosis. The results of in vivo studies demonstrate effects on enhancing intestinal barrier function, maintaining gut microbiota, and supporting cognitive health. (Manuscript in progress)
Live Cell Content
>1011 CFU/g; customized content on request
Daily Dosage
5-10 billion CFU (50-100 mg)
Appearance
white to light yellow powder
Package
1 kg/aluminum foil bag
Storage Condition and Shelf Life
2 years when sealed and stored at 4°C
Safety Tests
Patents
Patents granted in Taiwan
Scientific Support
In Submission








