
The Breakthrough of RYR, with Proven Clinical Trials and over 130 Publications for Metabolic Health
Market leadership in Taiwan probiotic markets
R&D Personnel Team
Leadership team with proven track record of driving success globally
Leadership team with proven track record of driving success globally
Vigiis101-LAB contains an excellent patented functional strain Lactobacillus paracasei subsp. paracasei NTU 101, which is a legally edible probiotic species in EU, Canada, China, and Taiwan. Safety and functions have been verified through plenty of scientific studies. It possesses strong tolerance to gastric acid and bile salts so there is no need for microencapsulation. As a food ingredient and/or dietary ingredient, its concentration is up to more than 100 billion CFU/g and very low water activity (< 0.1) contributes to its high stability. Thus, it is very suitable for formulation of dietary supplements and fortified foods.

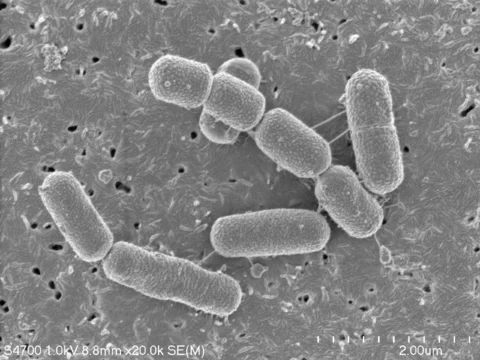
Lactobacillus paracasei subsp. paracasei NTU 101 is a patented functional strain of lactic acid bacteria, studied for more than ten years by the research team of Professor Tzu-Ming Pan from National Taiwan University. It is isolated from the intestinal microbial flora of breast-fed infants in 3 days after birth. Science basis is greatly backed by more than 40 related SCI publications, including many animal studies demonstrating the effects of this strain and its fermented products on enhancing digestion, improvement in gastrointestinal microbiota, modulation of immune system and allergic reaction, etc.
Live Cell Content
>1011 CFU/g; customized content on request
Daily Dosage
5-10 billion CFU (50-100 mg)
Appearance
light yellow powder
Package
1 kg / aluminum foil bag
Storage & Shelf Life
2 years when sealed and stored at -20°C
Safety Tests
Special Status
US FDA GRAS
Patents
Patents granted in USA, EU, Japan, China, Taiwan, Korea
Scientific Support
Backed by 40+ SCI publications including clinical and animal studies













